
Foundation and higher tiers
There are many ways of drawing covalent molecules with each method having its advantages and disadvantages. Often it depends on what you are trying to show or describe that will determine what type of drawing or representation to use e.g. different ways of drawing an ammonia molecule NH3 are shown below; each method of representing the ammonia molecule has its advantages and disadvantages:
The drawing below shows two dot and cross diagrams for an ammonia molecule. The only difference between them is one has the electron shells shown. It is entirely up to you as to whether you chose to draw in the electron shells, but it they can help in visualising the electron pairs in the covalent bonds.
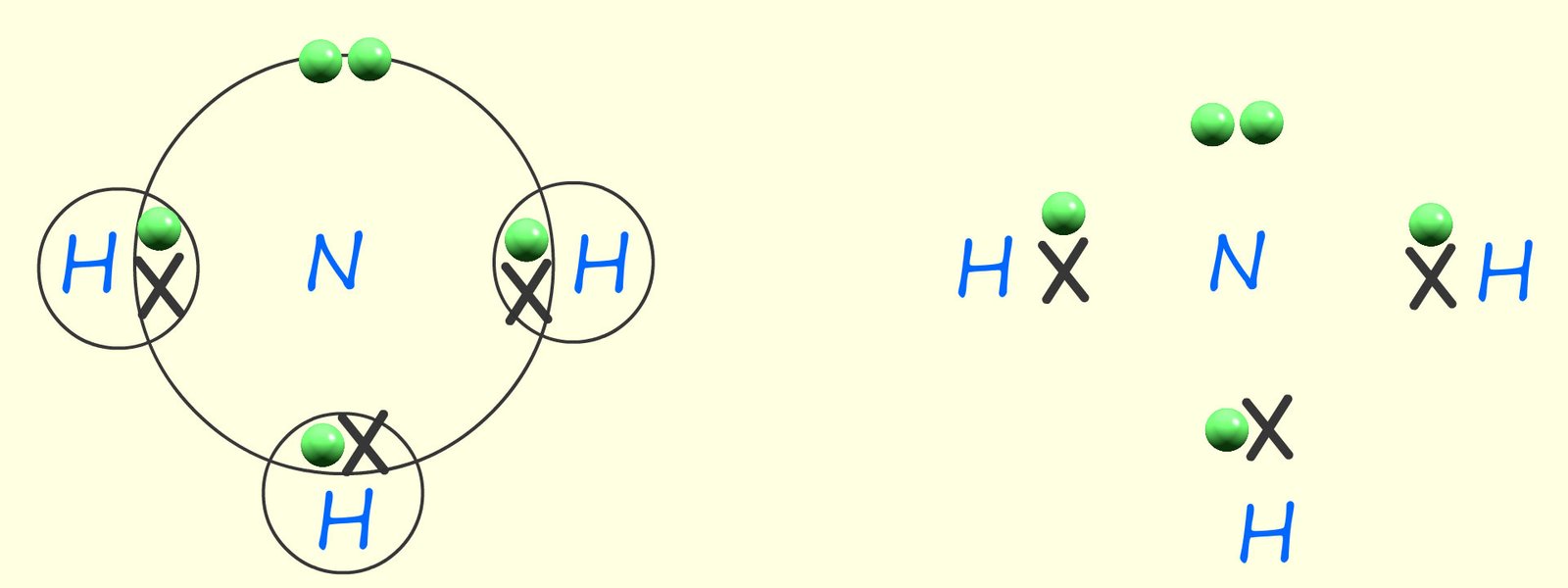
While dot and cross diagrams are helpful in showing the positions of the electrons in the molecule they give no indication of the shape of the molecule or its actual size.
The two drawing below show the displayed formula of the ammonia molecule, the solid black lines represent the covalent bonds within the molecule. The drawing on the left shows the ammonia molecule with its lone pair or non-bonding pair of electrons. Displayed formulae are useful in showing how the atoms in a particular molecule are connected but they give no indication of the shape of the molecule.
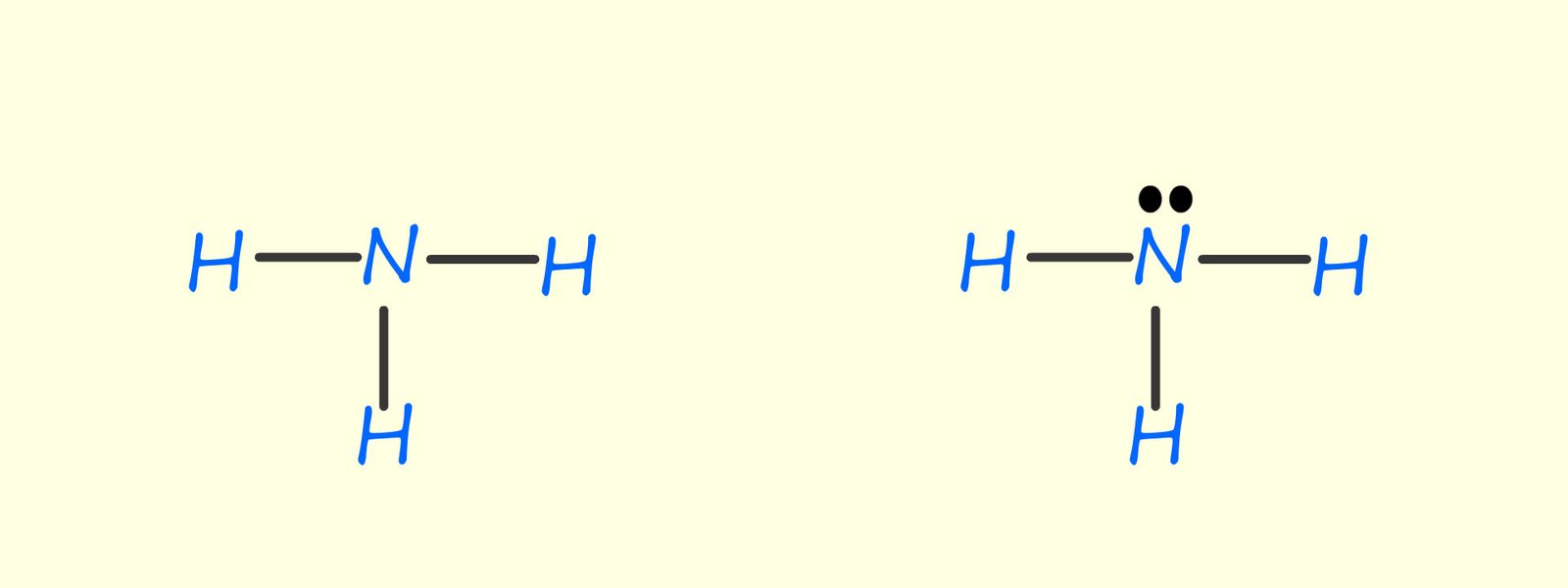
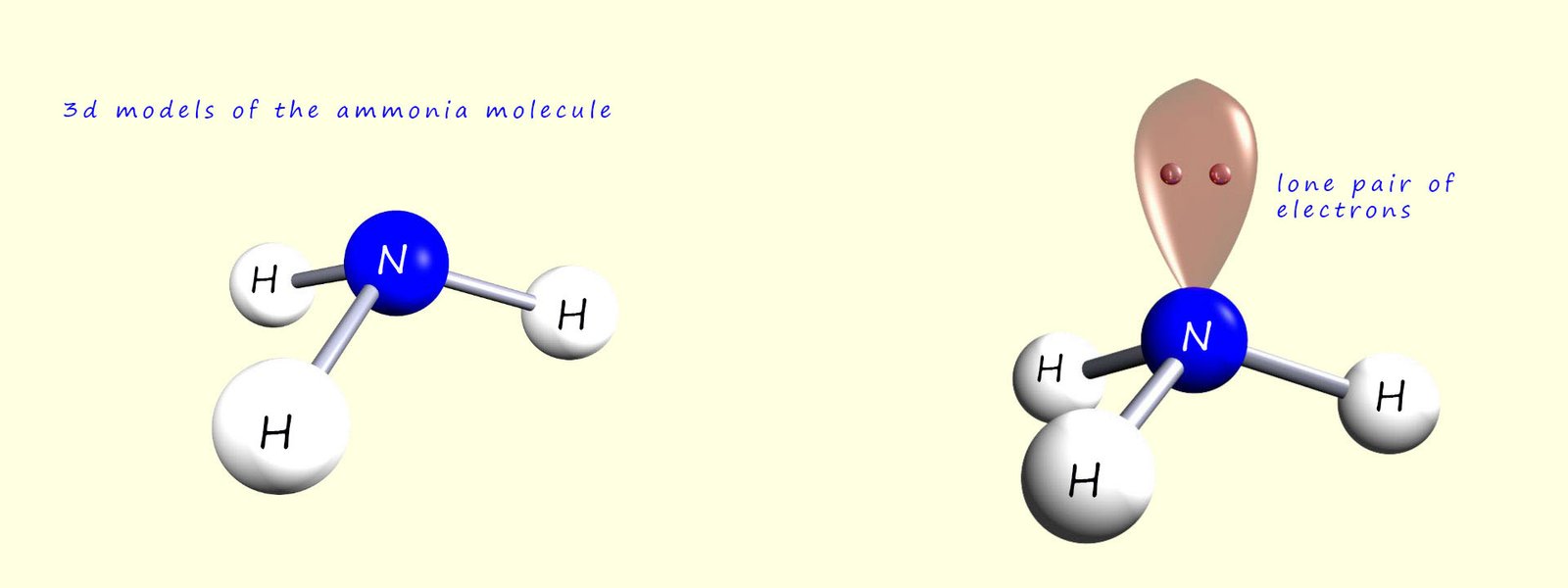
The image below shows 3d models of the ammonia molecule. 3d models are useful for showing the actual shape of the molecule but they give no detail on how the electrons are arranged in the molecule.